Introducing the Covalent Compound Naming Practice Answer Key, your ultimate resource for mastering the art of naming these chemical compounds. This comprehensive guide provides a step-by-step approach to understanding the principles and rules governing covalent compound nomenclature, empowering you with the knowledge to confidently name and identify these essential chemical entities.
Delving into the intricacies of covalent bonding, we will explore the characteristics that define covalent compounds and unravel the systematic approach to naming them. Prepare to embark on a journey of chemical literacy, where the complexities of covalent compound nomenclature are transformed into a comprehensible and accessible subject.
Introduction to Covalent Compounds
Covalent compounds are formed when atoms share electrons to achieve a stable electron configuration. The shared electrons form covalent bonds, which hold the atoms together.
Covalent compounds typically have the following characteristics:
- Low melting and boiling points
- Poor electrical conductivity
- Soluble in nonpolar solvents
Nomenclature of Covalent Compounds
Step-by-Step Guide to Naming Covalent Compounds
- Identify the central atom (the atom that is bonded to the most other atoms).
- Use the root of the element name for the central atom.
- Use prefixes to indicate the number of atoms of each element bonded to the central atom.
- Use the suffix “-ide” for the other atoms.
Use of Prefixes to Indicate the Number of Atoms, Covalent compound naming practice answer key
| Number of Atoms | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |
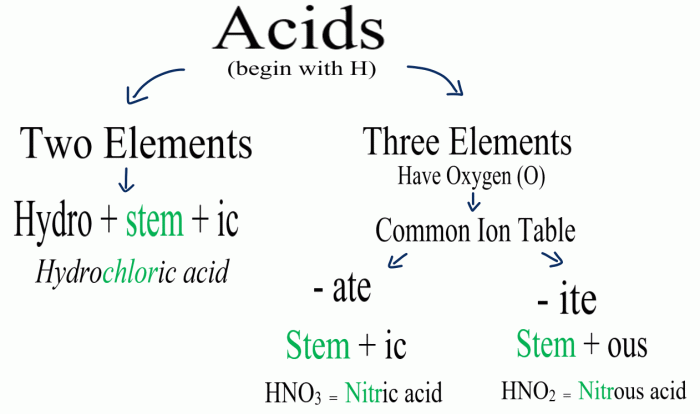
Rules for Naming Polyatomic Ions
Polyatomic ions are groups of atoms that have a net charge. They are named using the following rules:
- The root of the element name is used for the first element in the ion.
- The suffix “-ate” is used for ions with an oxygen atom.
- The suffix “-ite” is used for ions with an oxygen atom and a lower oxidation state.
- The suffix “-ide” is used for ions without an oxygen atom.
Practice Naming Covalent Compounds
| Molecular Formula | Name | Practice Questions |
|---|---|---|
| HCl | Hydrogen chloride | Name the compound with the molecular formula CH4. |
| CO2 | Carbon dioxide | Name the compound with the molecular formula NH3. |
| H2O | Water | Name the compound with the molecular formula N2O5. |
Answer Key:
- CH 4: Methane
- NH 3: Ammonia
- N 2O 5: Dinitrogen pentoxide
Advanced Topics in Covalent Compound Nomenclature: Covalent Compound Naming Practice Answer Key
Exceptions to the General Rules of Nomenclature
There are a few exceptions to the general rules of nomenclature. For example, the compound H 2O is called water, not dihydrogen oxide.
Use of Greek Prefixes for Large Numbers of Atoms
When a covalent compound contains a large number of atoms, Greek prefixes are used to indicate the number of atoms. For example, the compound C 10H 22is called decane.
Examples of Complex Covalent Compounds and Their Names
Complex covalent compounds can have very long names. For example, the compound Fe 2O 3·xH 2O is called iron(III) oxide hydrate.
FAQ Section
What is the primary purpose of the Covalent Compound Naming Practice Answer Key?
The Covalent Compound Naming Practice Answer Key provides a comprehensive resource for understanding the principles and rules governing the naming of covalent compounds.
How does this guide approach the topic of covalent compound nomenclature?
This guide adopts a systematic approach, breaking down the process of naming covalent compounds into manageable steps, making it accessible to learners of all levels.
What benefits can I expect from using the Covalent Compound Naming Practice Answer Key?
By utilizing this guide, you will gain a deep understanding of covalent compound nomenclature, enabling you to confidently name and identify these compounds in various chemical contexts.